Coupling of two curved polyaromatic radical-anions: stabilization of dimers by counterions†
Abstract
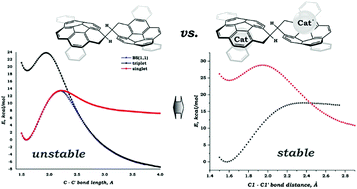
In this study, a comprehensive theoretical investigation of both kinetic and thermodynamic stabilities was performed for dimeric dianionic systems (C20H10)22− and (C28H14)22−, neutralized by two alkali metal cations. The influence of the counterions was of primary interest. The impact of the additional/spectator ligand(s) was elucidated by considering adducts with four molecules of diglyme or two molecules of 18-crown-6 ether. Importantly, both types of systems – in the form of contact-ion pair (CIP) and solvent-separated ion pair (SSIP) – were considered. The SSIP set was augmented by the adduct, in which the dimeric dianionic species were neutralized with purely organic cations N(CH3)4+ and P(CH3)4+. Detailed analysis of the bonding revealed that the presence of the counterions made these systems thermodynamically stable. This finding is in sharp contrast with results obtained for isolated (PAH)22− systems, which were previously found to be thermodynamically unstable, but kinetically persistent. The introduction of the alkali metal cations to the system significantly increases the ionic term (ΔEelstat), whereas the repulsive ΔEPauli one was found to be substantially reduced. Considering that the orbital component (ΔEorb) exhibited only a moderate decrease and the preparation energy (ΔEprep) showed no changes, the above-mentioned changes in ΔEelstat and ΔEPauli provided a clear explanation for the increase of the thermodynamic stability of the target species. Importantly, a clear correlation between the size of the alkali metal cation and stability of the target dimeric product was established. Thermodynamic stability of the system rises with a decrease in the size of M+ due to enlargement of the ΔEorb. Evaluated energy barriers (as spin-crossing points between singlet and triplet energy surfaces) were found to be equal to +15.85 kcal mol−1 and +18.5 kcal mol−1 for [(Cs+)2{(C20H10)22−}] and [(Cs+)2{(C28H14)22−}], respectively, which is substantially higher than those calculated for isolated (PAH)22− systems (+10.00 kcal mol−1 for (C20H10)22− and +12.35 kcal mol−1 for (C28H14)22−). Thus, this study identified the presence of counterions as the key factor, which have a dramatic influence on the thermodynamic and kinetic stabilities of the aimed dianionic dimeric systems, which are formed by two curved polyaromatic monoanion-radicals.


 Please wait while we load your content...
Please wait while we load your content...