Theoretical study on thermal curing mechanism of arylethynyl-containing resins†
Abstract
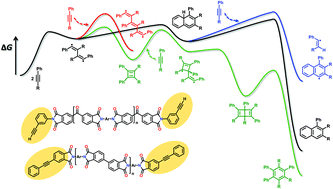
Arylethynyl reactive groups have been widely used in high-temperature polymers, and therefore, understanding their curing mechanism is of great importance for academic research and engineering applications. However, no consensus has been achieved on the actual curing mechanism of arylethynyl-containing resins so far. Herein, we present a density functional theory study on the thermal curing mechanism of arylethynyl-containing resins using phenylacetylene and diphenylacetylene as model compounds. It was discovered that the rate-determining step is the dimerization of arylacetylenes into diradical intermediates. The possibilities of the Straus-type intermediates and concerted Diels–Alder cycloaddition between two arylacetylenes can be ruled out. Cyclobutadiene and cyclic allene are the critical intermediates generated by the intramolecular coupling of diradicals. The formation of polyene is preferred by monoradical initiation rather than diradical growth. The overall reaction pathways can well account for the formation of naphthalenic dimers, benzenic trimers, and polyenic chains. The computational results of reactivity for the dimerization of arylacetylenes were finally compared with the existing experimental findings, and an agreement is shown.

 Please wait while we load your content...
Please wait while we load your content...