Structural interpretation of the energetic performances of a pure silica LTA-type zeolite†
Abstract
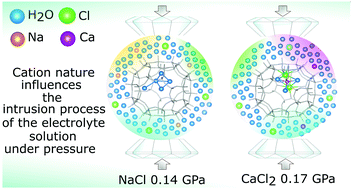
The high pressure intrusion–extrusion process of different electrolyte aqueous solutions (NaCl and CaCl2, 2 M and 3 M) in a hydrophobic pure-silica LTA zeolite was investigated for energetic purposes by means of in situ X-ray powder diffraction, porosimeter tests, thermogravimetric analysis and NMR spectroscopy. The intrusion pressure of the saline solutions was proved to be higher than that of pure water, with the highest value measured for CaCl2, thus increasing the energetic performance of the system. The intrusion of NaCl solutions was irreversible (bumper behavior), whereas that of CaCl2 solutions is partially reversible (shock absorber behavior). The structural investigation allowed interpreting these results on the basis of the different intrusion mechanisms, in turn induced by the different nature of the cations present in the electrolyte solutions. When Si-LTA is intruded by NaCl solution, firstly H2O molecules penetrate the pores, leading to higher silanol defect formation followed by the solvated ions. With CaCl2, instead, due to a higher solvation enthalpy of Ca2+, a higher pressure is required for intrusion, and both H2O and ions penetrate at the same pressure. The structural refinements demonstrate (i) a different arrangement of the extraframework species in the two systems, (ii) the intrusion of the salt solutions occurs through strong desolvation of the ions and (iii) the salt/H2O ratios of the intruded species are higher than those of the starting electrolyte solutions.


 Please wait while we load your content...
Please wait while we load your content...