H/D solvent isotope effects on the photoracemization reaction of enantiomeric the tris(2,2′-bipyridine)ruthenium(ii) complex and its analogues†
Abstract
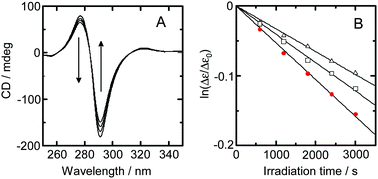
This work assessed solvent isotope effects on the photoracemization rate and emission lifetime for [Ru(bpy)3]2+ (bpy = 2,2′-bipyridine) in water. An analysis of the effects of temperature on photoracemization rate and emission lifetime demonstrated that the transition from one enantiomer to the other is unaffected by the isotopic composition of the solvent. The results also showed that deactivation from the metal-to-ligand charge-transfer (3MLCT) excited state to the ground state is responsible for the solvent isotope effect on the photoracemization rate. The photoracemization reaction was found to proceed via a bond-breaking mechanism. In this process, a five-coordinated species produced through breaking of the Ru–N bond in the 3d–d* state undergoes a structural change to produce an achiral five-coordinated species. An analysis of the effect of temperature on emission lifetime, excluding the activation to the 3d–d* state that leads to the structural change, showed that the solvent isotopic composition affects deactivation from the 4th MLCT state.

 Please wait while we load your content...
Please wait while we load your content...