Isomer-specific cryogenic ion vibrational spectroscopy of the D2 tagged Cs+(HNO3)(H2O)n=0–2 complexes: ion-driven enhancement of the acidic H-bond to water†
Abstract
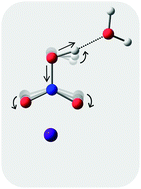
We report how the binary HNO3(H2O) interaction is modified upon complexation with a nearby Cs+ ion. Isomer-selective IR photodissociation spectra of the D2-tagged, ternary Cs+(HNO3)H2O cation confirms that two structural isomers are generated in the cryogenic ion source. In one of these, both HNO3 and H2O are directly coordinated to the ion, while in the other, the water molecule is attached to the OH group of the acid, which in turn binds to Cs+ with its –NO2 group. The acidic OH stretching fundamental in the latter isomer displays a ∼300 cm−1 red-shift relative to that in the neutral H-bonded van der Waals complex, HNO3(H2O). This behavior is analyzed with the aid of electronic structure calculations and discussed in the context of the increased effective acidity of HNO3 in the presence of the cation.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...