Peculiar hydrogen bonding behaviour of water molecules inside the aqueous nanochannels of lyotropic liquid crystals†
Abstract
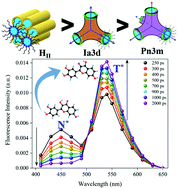
In spite of the widespread utilizations of lyotropic liquid crystals (LLCs) in food technology, as nanoreactors and in biomedical fields, the exact nature of their aqueous nanochannels which are deemed to dictate these applications are not completely understood. In this context, elucidation of the hydrogen bonding properties of the water molecules inside the nanochannels will contribute towards obtaining a complete picture of the LLC materials. In this study, we use two molecules exhibiting an excited state intramolecular proton transfer (ESIPT), fisetin and 3-hydroxyflavone, to determine the hydrogen bond donating and accepting parameters of the LLC water molecules. The steady state results imply a heterogeneity in the hydrogen bond accepting and donating properties inside the LLC nanochannels. Upon photoexcitation of the normal form of the ESIPT molecules, we notice that despite a reported general alcohol like polarity of the LLC nanochannels, the hydrogen bonding behaviour of the water molecules is similar to that of moderately polar aprotic solvents such as acetonitrile. In contrast, on excitation of the anionic species we observe that the spectral pattern is similar to that in alcohols. Additionally, the effect of the LLC water molecules on the rate of the intramolecular hydrogen transfer process has been explored. The ESIPT rates of both the probes, which are ultrafast (<20 ps) in neat polar protic and aprotic solvents, get slowed down dramatically by almost 15 times inside the LLC phases. Such an extent of retardation in the ESIPT rate is extremely rare in the literature, which signals towards the unique behaviour of the water molecules inside the LLC nanochannels. The structural topology of the LLC phases also influences the ESIPT rate with the timescale of the process increasing from the cubic to the hexagonal phase.

 Please wait while we load your content...
Please wait while we load your content...