Understanding the enhanced photoelectrochemical water oxidation over Ti-doped α-Fe2O3 electrodes by electrochemical reduction pretreatment†
Abstract
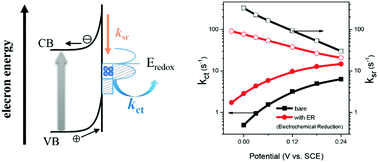
Water splitting using semiconductor photoelectrodes is a promising approach to solar hydrogen production. Previous studies have well-demonstrated that electrochemical reduction (ER) pretreatment of bare and Ti-doped α-Fe2O3 electrodes enhances water photooxidation efficiencies, however, the mechanism underlying this improvement remains poorly understood. In this study, this was quantitatively investigated by multiple photoelectrochemical techniques and transient absorption spectroscopy, using the doped electrodes as examples. The results reveal that the kinetics of photoholes after moving to the electrode surface can be well described by a model of surface-state mediated charge transfer and recombination. The reason for the photocurrent enhancement is attributed to a significantly increased charge transfer rate constant (kct) and a decreased surface recombination rate constant (ksr) by ER. The reason for the accelerated kct is that a new type of surface state, with a favorable energy position for water oxidation, is produced. The decreased ksr is due to the reduced electron density at the surface of the semiconductor, resulted predominately from the negatively shifted flat band potential. These findings provide new insights into the mechanism of water photooxidation and enlighten a simple way to develop more efficient electrodes.


 Please wait while we load your content...
Please wait while we load your content...