Preferential solvation of p-nitroaniline in a binary mixture of chloroform and hydrogen bond acceptor solvents: the role of specific solute–solvent hydrogen bonding†
Abstract
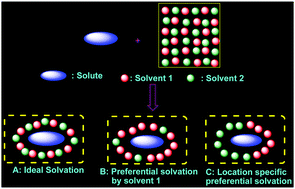
Preferential solvation of solvatochromic probe p-nitroaniline (PNA) has been studied in binary mixtures of chloroform with different hydrogen bond acceptor (HBA) solvents using the spectroscopic transition energy (ET). Analyses of the solvatochromic shifts of the absorption spectra of PNA in different neat solvents as a function of the solvent polarity parameters reveal the major contribution from the solvent dipolarity/polarizability and HBA basicity in the solvation of PNA. The event of preferential solvation in the chloroform–HBA binary mixtures and the preference of one solvent above the other in the solvation shell have been attributed to the hydrogen bond donor and acceptor ability of the solvent mixtures. HBA solvents form a strong hydrogen bond with the amino group while chloroform forms a hydrogen bond with the nitro group of PNA. This specific functional group recognition increases the local concentration at specific sites resulting in location specific preferential solvation and synergistic preferential solvation. Solvents with comparable polarity have been found to show significant synergistic behavior as a result of the formation of stronger solvent–solvent hydrogen bonded S1–S2 species. These propositions were found to be supported by theoretical solvation models, calculated HOMO–LUMO energy gaps, the effect of deuterated solvent on the extent of PS, and 1H NMR spectral analyses.


 Please wait while we load your content...
Please wait while we load your content...