A new methodology for a detailed investigation of quantized friction in ionic liquids†
Abstract
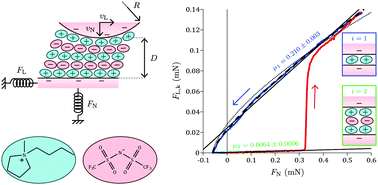
When confined at the nanoscale between smooth surfaces, an ionic liquid forms a structured film responding to shear in a quantized way, i.e., with a friction coefficient indexed by the number of layers in the gap. So far, only a few experiments have been performed to study this phenomenon, because of the delicate nature of the measurements. We propose a new methodology to measure friction with a surface force balance, based on the simultaneous application of normal and lateral motions to the surfaces, allowing for a more precise, comprehensive and rapid determination of the friction response. We report on proof-of-concept experiments with an ionic liquid confined between mica surfaces in dry or wet conditions, showing the phenomenon of quantized friction with an unprecedented resolution. First, we show that the variation of the kinetic friction force with the applied load for a given layer is not linear, but can be quantitatively described by two additive contributions that are respectively proportional to the load and to the contact area. Then, we find that humidity improves the resistance of the layers to be squeezed-out and extends the range of loads in which the liquid behaves as a superlubricant, interpreted by an enhanced dissolution of the potassium ions on the mica leading to a larger surface charge. There, we note a liquid-like friction behavior, and observe in certain conditions a clear variation of the kinetic friction force over two decades of shearing velocities, that does not obey a simple Arrhenius dynamics.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...