Stability and metallization of solid oxygen at high pressure†
Abstract
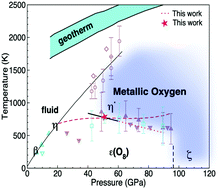
The phase diagram of oxygen is investigated for pressures from 50 to 130 GPa and temperatures up to 1200 K using first-principles theory. A metallic molecular structure with the P63/mmc symmetry (η′ phase) is determined to be thermodynamically stable in this pressure range at elevated temperatures above the ε(O8) phase. Crucial for obtaining this result is the inclusion of anharmonic lattice dynamics effects and accurate calculations of exchange interactions in the presence of thermal disorder. We present analysis of electronic, structural, and thermodynamic properties of solid oxygen at 0 K and finite temperature with hybrid exchange functionals, including a comparison with available experimental data.


 Please wait while we load your content...
Please wait while we load your content...