Velocity map imaging study of the photodissociation dynamics of the allyl radical†
Abstract
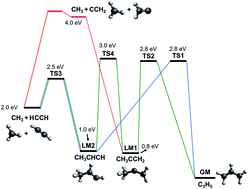
The photodissociation of the allyl radical (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2˙) following excitation between 216 and 243 nm has been investigated employing velocity map imaging in conjunction with resonance enhanced multiphoton ionization to detect the hydrogen atom and CH3(ν = 0) produced. The translational energy distributions for the two fragments are reported and analyzed along with the corresponding fragment ion angular distributions. The results are discussed in terms of the different reactions pathways characterizing the hydrogen atom elimination and the minor methyl formation. On one hand, the angular analysis provides evidence of an additional mechanism, not reported before, leading to prompt dissociation and fast hydrogen atoms. On the other hand, the methyl elimination channel has been characterized as a function of the excitation energy and the contribution of three reaction pathways: single 1,3-hydrogen shift, double 1,2-hydrogen shift and through the formation of vinylidene have been discussed. Contrary to previous predictions, the vinylidene channel, which plays a significant role at lower energies, seems to vanish following excitation on the Ẽ2B1(3px) excited state at λ ≤ 230 nm.
CH–CH2˙) following excitation between 216 and 243 nm has been investigated employing velocity map imaging in conjunction with resonance enhanced multiphoton ionization to detect the hydrogen atom and CH3(ν = 0) produced. The translational energy distributions for the two fragments are reported and analyzed along with the corresponding fragment ion angular distributions. The results are discussed in terms of the different reactions pathways characterizing the hydrogen atom elimination and the minor methyl formation. On one hand, the angular analysis provides evidence of an additional mechanism, not reported before, leading to prompt dissociation and fast hydrogen atoms. On the other hand, the methyl elimination channel has been characterized as a function of the excitation energy and the contribution of three reaction pathways: single 1,3-hydrogen shift, double 1,2-hydrogen shift and through the formation of vinylidene have been discussed. Contrary to previous predictions, the vinylidene channel, which plays a significant role at lower energies, seems to vanish following excitation on the Ẽ2B1(3px) excited state at λ ≤ 230 nm.


 Please wait while we load your content...
Please wait while we load your content...