Water dissociation and hydrogen evolution on the surface of Fe-based bulk metallic glasses†
Abstract
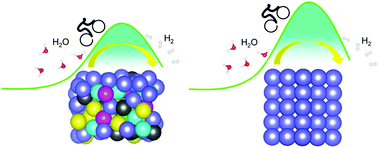
Fe-Based bulk metallic glasses (BMGs) with a composition of Fe48Cr15Mo14C15B6Y2 have recently been reported with good application performance due to their excellent mechanical and chemical properties, showing excellent corrosion resistance, remarkable forming and processing ability, ultrahigh yield strength and large elasticity. Here, we report on a new functional application for such Fe-based BMGs, which can exhibit better catalytic performance than the pristine Fe surface. The hydrogen evolution and dissociation processes of one and two H2O molecules on both BMG and pristine Fe surfaces are investigated using first-principles calculations. The energy barriers of the dissociation processes on the BMG surface are lower than those on the pristine Fe surface. Moreover, the structural configurations along the dissociation path during hydrogen evolution show that it is easier for H2O molecules to dissociate into H2 on the surface of the BMG, rendering it a more active catalyst than the pristine Fe surface. Analyses on the electronic structures show further evidence that the BMG surface has a stronger ability to facilitate charge transfer at the interface and is more inclined to accept transferred charges, thereby promoting its catalytic activity. These findings shed light on understanding the functional applications of BMGs and are anticipated to be highly meaningful for further experimental investigations.


 Please wait while we load your content...
Please wait while we load your content...