Crystal engineering construction of caffeic acid derivatives with potential applications in pharmaceuticals and degradable polymeric materials†
Abstract
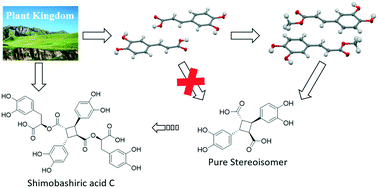
Natural products are precious feedstock in drug discovery and sustainable materials. This work using crystal engineering strategy, visible light, and solvent-free cycloaddition successfully constructed two caffeic acid derivatives, rel-(1R,2R,3S,4S)-2,4-bis(3,4-dihydroxyphenyl)cyclobutane-1,3-dicarboxylate and rel-(1R,2R,3S,4S)-2,4-bis(3,4-dihydroxyphenyl)cyclobutane-1,3-dicarboxylic acid. Because of the multiple stereocenters, it is challenging to prepare those compounds using traditional organic synthesis methods. The crystal engineering Hirshfeld surface analysis and 2D intermolecular interaction fingerprints were applied to synthetic route design. The light resources used in this work was visible LED or free, clean, and renewable sunlight. The evidence suggested that pure stereoisomer was obtained demonstrating the stereospecificity and efficiency of the topochemical cycloaddition reaction. The derivatives exhibited free radical scavenging and antioxidant biological activities, as well as the potential inhibitory activity of fatty acid binding proteins. One of the derivatives is the precursor of the natural product shimobashiric acid C which paves the way for the total synthesis and further study of shimobashiric acid C. In addition, the derivatives possess photodegradability at a specific wavelength, which is very attractive for “green” degradable polymeric materials.


 Please wait while we load your content...
Please wait while we load your content...