A new dipeptide as a selective gelator of Cu(ii), Zn(ii), and Pb(ii)†
Abstract
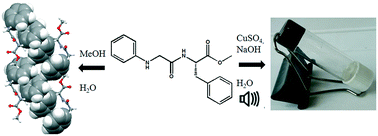
The various aspects associated with the incorporation of steric constraints into a series of dipeptides have been studied. The self-assembly propensities of the dipeptides carried out with the use of complementary experimental methods enabled us to determine the effect of steric constraints. Gly and Aib containing peptide 1 shows a flake-like morphology. The replacement of Gly by L-Phe in peptide 2 increases the steric constraints and results in development of microrods. Peptide 3 with N-phenylglycine and L-Phe has a flower-like morphology, and peptide 4 with N-phenylglycine and L-Tyr has a microsphere morphology. The solid-state FT-IR studies show that the peptides self-assemble by hydrogen bonding. From X-ray crystallography, peptide 1 adopts a kink-like conformation and forms a supramolecular anti-parallel sheet-like structure through multiple intermolecular hydrogen bonds. The replacement of Gly by L-Phe helps peptide 2 to adopt an S-shape conformation and self-assemble to form a supramolecular helix in the solid state. Single crystal X-ray diffraction results exhibit that peptide 3 adopts an extended conformation and forms a 2D sheet-like matrix through π–π stacking interactions. Moreover, metallogelation of peptide 3 was observed selectively for CuSO4·5H2O, ZnSO4·7H2O and Pb(OAc)2·3H2O, whereas other metals were not able to form gel. This study revealed a pivotal role played by the phenyl group in the diverse self-assembly of dipeptides.


 Please wait while we load your content...
Please wait while we load your content...