Direct salinization of trelagliptin from solid forms by mechanochemistry and its mechanism of salt formation†
Abstract
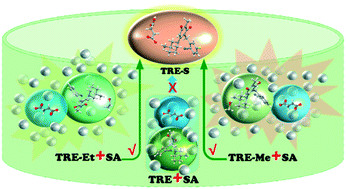
Preparing active pharmaceutical ingredients (APIs) by designing methods with the concept of “green production” is challenging. In this study, an efficient mechanochemical salinization method was developed to prepare trelagliptin succinate (TRE-S), the first once-weekly oral treatment drug for type II diabetes. Trelagliptin (TRE), trelagliptin ethanol solvate (TRE-Et), trelagliptin methanol solvate (TRE-Me), and TRE-S were prepared and characterized via different analytical techniques. Various trelagliptin solid forms were assessed to verify the efficacy of forming TRE-S by the milling method. Intriguingly, TRE-S could be obtained by milling trelagliptin solvates and succinic acid (SA) with a mole ratio of 1 : 1. However, only amorphous solid forms were obtained by milling the mixture of TRE and SA. Liquid-assisted grinding of TRE and SA with ethanol or methanol could also prepare TRE-S. We have attempted to unearth the reaction mechanism for the formation of TRE-S via the ball-milling method. This work illustrates that similar intermolecular interactions in crystals and any factor inducing proton transfer have a vital role in the formation of API salts by mechanochemistry.


 Please wait while we load your content...
Please wait while we load your content...