Can solvated intermediates inform us about nucleation pathways? The case of β-pABA†
Abstract
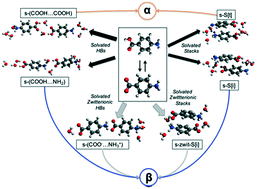
Classical nucleation theory teaches the idea that molecular clusters form and grow in solution and that depending on prevailing conditions there is a chance for some to grow large enough to overcome the interfacial energy penalty and become mature crystals. However, from such a kinetic analysis, nothing is learnt of the nature of the composition or the molecular packing in such clusters. As a means of addressing this shortcoming consideration has, in the past, been given to the idea that in certain systems crystallography may offer additional, structural, insights. From this approach the notions of ‘nucleation pathway’ or ‘nucleation transition state’ have become useful concepts around which to formulate hypotheses as to how clusters may yield specific molecular packing, resulting for example, in the observation of crystal polymorphs. Here we offer an in-depth crystallographic analysis related to the nucleation of the α and β polymorphs of para-aminobenzoic acid in an attempt to reveal the pathways leading to the two forms. Using a combination of CSD analyses, crystal structure prediction and targeted crystallizations we explore plausible solution pathways to these polymorphs and discuss our results in the light of known kinetic data for the nucleation and growth of this material.
- This article is part of the themed collection: The Cambridge Structural Database - A wealth of knowledge gained from a million structures


 Please wait while we load your content...
Please wait while we load your content...