A novel lurasidone hydrochloride–shikimic acid co-amorphous system formed by hydrogen-bonding interaction with the retained pH-dependent solubility behavior†
Abstract
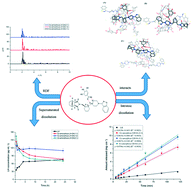
In this work, we report on a novel lurasidone hydrochloride (LH)–shikimic acid (SA) co-amorphous system (LH–SA). It was formed via hydrogen-bonding interaction between LH and SA using a solvent evaporation method. The formation of the LH–SA co-amorphous system was confirmed by the powder X-ray diffraction and differential scanning calorimetry. The intermolecular interaction between LH and SA was explored by FTIR, Raman spectroscopy and molecular dynamic simulation. Interestingly, we found the pH-dependent solubility behavior of the co-amorphous system was retained in the pH range of 1.0–6.8, suggesting that the hydrogen-bonding interaction might not be the main reason for restoring the pH-dependent solubility behavior of LH. The solubility and dissolution rates of the co-amorphous LH–SA system were remarkably improved in comparison with the crystalline LH. We believe that the co-amorphous system might show great potential in enhancing the oral bioavailability of LH.


 Please wait while we load your content...
Please wait while we load your content...