Co-crystallization of racemic amino acids with ZnCl2: an investigation of chiral selectivity upon coordination to the metal centre†
Abstract
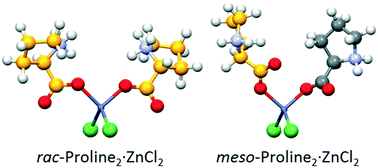
The amino acids alanine, valine, proline, isoleucine, serine, asparagine, tyrosine, and threonine in their racemic forms have been reacted with ZnCl2 under different preparative conditions (grinding and LAG, both manual and ball milling, and co-crystallization from solvent). In most cases, relatively stable (from days to months) oils were obtained; only in those cases for which single crystals could grow from oils, structural characterisation was possible via X-ray diffraction. Aim of the work has been the investigation of the occurrence of chiral selectivity upon the formation of tetrahedral metal coordination complexes or polymers. It has been shown that co-crystallization reactions lead, in the majority of cases, to crystals of racemic-AA2ZnCl2, formed by 0D homochiral complexes of formula L-AA2ZnCl2 and D-AA2ZnCl2. With the DL-amino acid threonine, however, crystals of meso-AA2ZnCl2 have also been obtained, made of 0D heterochiral complexes of formula DL-AA2ZnCl2. With DL-proline, both the known racemic and the new meso-AA2ZnCl2 solids were obtained. Formation of 1D coordination polymers has been observed in the cases of DL-asparagine and DL-tyrosine with alternating D and L amino acids along the polymeric chain.


 Please wait while we load your content...
Please wait while we load your content...