Crystal structure determination of an elusive methanol solvate – hydrate of catechin using crystal structure prediction and NMR crystallography†
Abstract
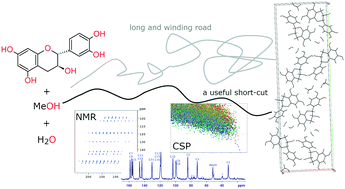
Experimental screening for new crystalline forms of a flavan-3-ol derivative, catechin, yielded several new solvates and solvate–hydrates of this polyphenol, among which a new methanol-containing crystalline solid was identified. This form was found to be different from the previously described 2 : 1 methanol solvate of catechin. It contains one molecule of water and 0.5 molecules of methanol per catechin molecule in the asymmetric unit. To determine the crystal structure of this form, NMR crystallography and crystal structure prediction (CSP) calculations were used, as every attempt to obtain a single crystal of sufficient quality to perform single crystal X-ray diffraction failed. To deal with a system containing five independent components in the CSP search (due to the necessity of treating whole molecules), in addition to the intramolecular flexibility of catechin with 68 viable conformers, we developed a useful short-cut, which allows for limiting the number of conformations considered in such demanding calculations. In this work, we show that it is possible to use the simulated NMR data for CSP-generated crystal structures of a simpler, yet similar system, to indicate the likely molecular conformation present in a more complex system. This approach allowed us to determine the experimental crystal structure of catechin methanol hemisolvate – monohydrate.


 Please wait while we load your content...
Please wait while we load your content...