Drug–drug cocrystals of anticancer drugs erlotinib–furosemide and gefitinib–mefenamic acid for alternative multi-drug treatment†
Abstract
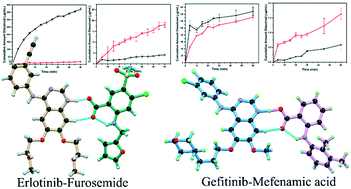
Drug–drug cocrystals of anticancer drugs erlotinib and gefitinib with furosemide and mefenamic acid, respectively, have been synthesized. The 1 : 1 erlotinib–furosemide cocrystal crystallizes in the monoclinic centrosymmetric P21/n space group containing one molecule of each component in the asymmetric unit. In contrast, the 1 : 1 gefitinib–mefenamic acid cocrystal hydrate belongs to the monoclinic centrosymmetric P21/c space group comprising one molecule of both drugs along with one water molecule in the asymmetric unit. The solubility and dissolution rate study revealed higher solubility for BCS class II drugs, furosemide, and mefenamic acid, while the solubility and dissolution rate of erlotinib showed a significant reduction in the cocrystal salt. Conversely, the solubility of gefitinib didn't reveal a substantial decrease; however, the dissolution rate has been reduced in the cocrystal hydrate. Further, an attempt has been made to correlate the crystal structures of the erlotinib–furosemide and gefitinib–mefenamic acid cocrystals with their solubilities and dissolution rate.


 Please wait while we load your content...
Please wait while we load your content...