Sun, UV and X-ray triple photochromic properties of three coordination polymers based on 1,1′-bis(3-carboxylatobenzyl)-4,4′-bipyridinium ligand†
Abstract
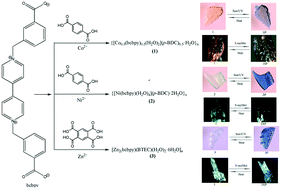
Based on a bipyridinium carboxylate ligand 1,1′-bis(3-carboxylatobenzyl)-(4,4′-bipyridinium)-dichloride (H2bcbpyCl2), three new coordination polymers {[Co0.5(bcbpy)0.5(H2O)2](p-BDC)0.5·H2O}n (1) {[Ni(bcbpy)(H2O)4](p-BDC)·2H2O}n (2) and [Zn2(bcbpy)(BTEC)(H2O)2·6H2O]n (3) are designed and synthesized by varying the benzenecarboxylic acids (1,4-benzenedicarboxylic acid = p-H2BDC, 1,2,4,5-benzenetetracarboxylic acid = H4BTEC) to adjust the coordination mode between the viologen ligand and metal ions. Single crystal structure analyses reveal that compounds 1 and 2 both exhibit one-dimensional (1D) chain-like structure, while compound 3 exhibits two-dimensional (2D) layered architecture. Furthermore, due to the formation of viologen radicals, reversible color changes for three compounds can be observed in the air upon sunlight, UV-light and X-ray irradiation. Particularly, compound 3 has high photosensitivity, giving an eye-detectable color change after exposure to sunlight in air within 1 min. In addition, the photoproducts for the three compounds exhibit an ultralonglived charge-separated state.


 Please wait while we load your content...
Please wait while we load your content...