A new 1 : 1 cocrystal of lamotrigine and 1,2,3,6-hydrophthalimide: discovery, characterization, and construction of ternary phase diagrams†
Abstract
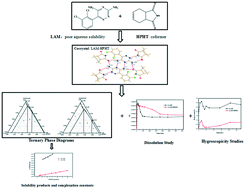
A 1 : 1 cocrystal of lamotrigine (LAM) and 1,2,3,6-hydrophthalimide (HPHT) was successfully synthesized for the first time and was characterized by XRD (powder and single-crystal X-ray diffraction), FT-IR, DSC and TG. Molecular Hirshfeld surfaces and the corresponding two-dimensional (2D) fingerprint plots have been evaluated and Hirshfeld surface analysis indicated that hydrogen bonds (N⋯H and O⋯H) mainly constitute the connection between the cocrystal molecules. The ternary phase diagrams (TPDs) of LAM–HPHT in isobutanol were constructed at 293.15 K and 313.15 K. The results indicated that as the temperature increases, the pure LAM–HPHT cocrystal preparation region became larger without changing TPD shape. Meanwhile, the solubility of LAM–HPHT in isobutanol was measured, and the solubility product (Ksp) and solution complexation (K11) constants were proposed to illustrate the solubility of LAM–HPHT. The result indicated that Ksp gradually increases while K11 decreases with the cocrystal solubility increasing. The dissolution results indicated that LAM–HPHT was found to exhibit improved aqueous solubility when compared to pure LAM. The stability study demonstrated that LAM–HPHT was much more stable than the original LAM crystal under the condition of 98% RH (25 °C) or 85% RH (25 °C).


 Please wait while we load your content...
Please wait while we load your content...