Effect of urea additives on CuSO4·5H2O crystal habit: an experimental and theoretical study
Abstract
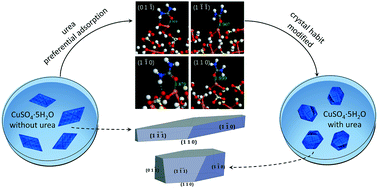
The effect of urea on the habit of copper(II) sulfate pentahydrate crystal and its mechanism has been studied. The drastic retardation of the (0 1 ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) face was induced by an additive modifying the crystal from a parallelogram to an oblique hexagonal prism. Two opposite effects caused by urea clearly revealed the synergistic impact of thermodynamic and kinetic factors. Density functional theory calculations have been performed to obtain the geometries and adsorption energies of water and urea molecules on (0 1
) face was induced by an additive modifying the crystal from a parallelogram to an oblique hexagonal prism. Two opposite effects caused by urea clearly revealed the synergistic impact of thermodynamic and kinetic factors. Density functional theory calculations have been performed to obtain the geometries and adsorption energies of water and urea molecules on (0 1 ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), (1
), (1 ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), (1
), (1 ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) and (1 1 0) faces. It could be inferred from the calculated geometries that the formation of the H2O–Cu bond was hampered by the binding of urea to Cu atoms, and the significant distinction (0.85 eV) of the adsorption energies suggests a preferential interaction on the (0 1
0) and (1 1 0) faces. It could be inferred from the calculated geometries that the formation of the H2O–Cu bond was hampered by the binding of urea to Cu atoms, and the significant distinction (0.85 eV) of the adsorption energies suggests a preferential interaction on the (0 1 ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) face. This work explains the mechanism rationally, and is helpful for evaluating habit modifiers under the guidance of theoretical calculations.
) face. This work explains the mechanism rationally, and is helpful for evaluating habit modifiers under the guidance of theoretical calculations.


 Please wait while we load your content...
Please wait while we load your content...