Stabilization of hydrogen peroxide by hydrogen bonding in the crystal structure of 2-aminobenzimidazole perhydrate†
Abstract
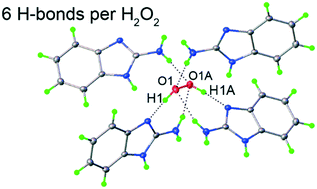
2-Aminobenzimidazole hemiperoxosolvate 2(C7H7N3)·H2O2 was synthesized and studied by single crystal X-ray analysis and periodic (solid-state) DFT calculations. The obtained compound, after urea and melamine peroxosolvates, is the third example of an H2O2 crystalline adduct stabilized with the maximum possible number of hydrogen bonds formed by one hydrogen peroxide molecule – 2 H-bonds as proton donors and 4 as acceptors. Due to the small size of the hydrogen peroxide molecule, its hydrogen bonding energy contributes the maximal impact and determines the relative value of the hydrogen bonding energy of the peroxosolvate crystal and can be suggested as an energetic criterion of perhydrate stability. The total energy of the 6 hydrogen bonds formed by one hydrogen peroxide molecule in all three compounds (∼140–∼170 kJ mol−1) was calculated and compared to the corresponding values for crystalline hydrogen peroxide and L-serine peroxosolvate. The total energy of the 4 hydrogen bonds of hydrogen peroxide molecule in crystalline H2O2 and L-serine peroxosolvate (150 and 113 kJ mol−1, respectively) was evaluated by solid-state DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...