Role of substituents on resonance assisted hydrogen bonding vs. intermolecular hydrogen bonding†
Abstract
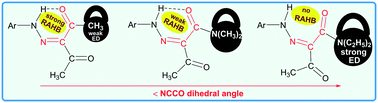
The role of substituents on resonance assisted hydrogen bonding (RAHB) vs. intermolecular hydrogen bonding is highlighted in known arylhydrazones of active methylene compounds (AHAMCs) and in their new representatives – (E/Z)-2-(2-(para-substitutedphenyl)hydrazineylidene)-N,N-diethyl-3-oxobutanamides (–C2H5, –H, –COOH, –CN). The strength of the H-bond depends on the attached functional groups to both the active methylene fragment and the aromatic moiety of the AHAMC. For instance, attachment of the strong electron donor group –N(C2H5)2 (σp = −0.72) to the active methylene fragment of AHAMC allows to isolate this class of compounds without RAHB but with strong intermolecular H-bonds, which are the first examples reported herein.


 Please wait while we load your content...
Please wait while we load your content...