Morphology evolution, energy transfer and multicolor luminescence of lanthanide-doped Ba2LaF7 nanocrystals via a one-step hydrothermal synthesis†
Abstract
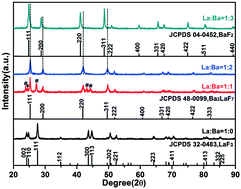
Lanthanide-doped Ba2LaF7 nanocrystals were successfully synthesized via a one-step hydrothermal method. The phase, size and shape of the lanthanide-doped Ba2LaF7 nanocrystals were systematically investigated by controlling the fluorine source, additive, lanthanide doping, synthesis method and temperature. Regular hexagonal Ba2LaF7 nanocrystals with a side length of 60 nm were obtained by using NaBF4 as the fluorine source. A variety of lanthanide ions (Ho3+, Er3+, Tm3+, Dy3+, Eu3+, Tb3+, Ce3+, and Sm3+) was successfully doped into the Ba2LaF7 host, which showed abundant luminescent colors due to the intrinsic emission of the lanthanide ions. Because of the emission spectrum of Tb3+ (5D4 → 7Fj) having obvious overlap with the Eu3+ excitation spectrum (7F0,1 → 5D0,1,2), the excited state energy of Tb3+ can be transferred to Eu3+. Under ultraviolet excitation, the energy transfer from Tb3+ to Eu3+ in the Ba2LaF7 matrix occurs mainly via the dipole–quadrupole mechanism. The regulation of luminescent color was achieved from green to warm yellow to yellow orange and finally to red by changing the doping concentration of Eu3+ ions from 0.1% to 7%, respectively. The obtained Ba2LaF7:Ln3+ nanocrystals have potential application in multicolor lighting and displays.


 Please wait while we load your content...
Please wait while we load your content...