Heteromolecular compounds in binary systems of amino acids with opposite and same chiralities†
Abstract
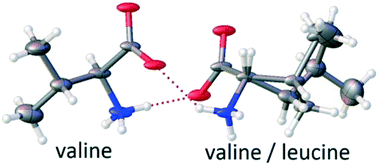
A classification of discrete compounds in binary chiral systems of single and different substances (homo- and heteromolecular compounds) is presented. It considers both chemical and crystallographic characteristics of such compounds. Using amino acids as chiral model systems, features of crystal structures of equimolar and non-equimolar heteromolecular compounds are reviewed. In this connection, the concept of homo- and heteromolecular dimers in compounds formed by amino acids is introduced and analyzed. As a result, a correlation between the molecular dimer type and the side chain structure (linear or branched) and the conformation (extended or folded) of the relevant compounds' molecules is derived. Two non-equimolar discrete heterocompounds discovered in the chiral systems L-valine–L-isoleucine and L-valine–L-leucine are discussed using the concepts proposed. The previously studied first system features a 2 : 1 (Val : Ile) compound. A newly investigated second system is found to form a 3 : 1 (Val : Leu) compound, and its crystal structure is described and evaluated.


 Please wait while we load your content...
Please wait while we load your content...