Structure-guided engineering of Pseudomonas dacunhael-aspartate β-decarboxylase for l-homophenylalanine synthesis†
Abstract
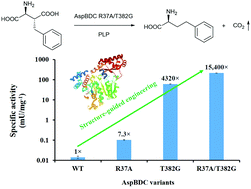
Structure-guided engineering of Pseudomonas dacunhaeL-aspartate β-decarboxylase (AspBDC) resulted in a double mutant (R37A/T382G) with remarkable 15 400-fold improvement in specific activity reaching 216 mU mg−1, towards the target substrate 3(R)-benzyl-L-aspartate. A novel strategy for enzymatic synthesis of L-homophenylalanine was developed by using the variant as a biocatalyst affording 75% product yield within 12 h. Our results underscore the potential of engineered AspBDC for the biocatalytic synthesis of pharmaceutically relevant and value added unnatural L-amino acids.


 Please wait while we load your content...
Please wait while we load your content...