A practical base mediated synthesis of 1,2,4-triazoles enabled by a deamination annulation strategy†
Abstract
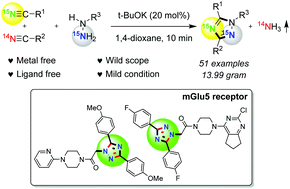
A rapid and efficient base mediated synthesis of 1,3,5-trisubstituted 1,2,4-triazoles has been developed using the annulation of nitriles with hydrazines, which can be expanded to a wide range of triazoles in good to excellent yields. Ammonia gas is liberated during the reaction, and halo and hetero functional groups as well as free hydroxyl and amino groups are tolerated in this transformation. A variety of alkyl and aryl-substituted nitriles can be functionalized with aromatic and aliphatic hydrazines employing this procedure. This finding provides a practical and useful strategy for the synthesis of various 15N-labeled 1,2,4-triazole derivatives, and two types of mGlu5 receptor pharmaceuticals can be easily assembled in a one-pot manner.


 Please wait while we load your content...
Please wait while we load your content...