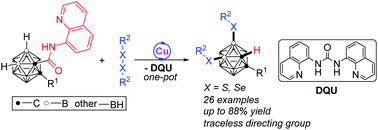
8-Aminoquinoline as a bidentate traceless directing group for Cu-catalyzed selective B(4,5)–H disulfenylation of o-carboranes†‡
Abstract
A traceless bidentate directing group guided copper catalyzed direct cage B(4,5)–H disulfenylation of o-carboranes has been achieved, leading to a series of B(4,5)–disulfenylated o-carborane derivatives in high yields with excellent regioselectivity. The in situ departure of the 8-aminoquinoline bidentate auxiliary circumvents additional processes for directing group removal, thus enhancing the atom-/step-economy.


 Please wait while we load your content...
Please wait while we load your content...