Target identification of a macrocyclic hexaoxazole G-quadruplex ligand using post-target-binding visualization†
Abstract
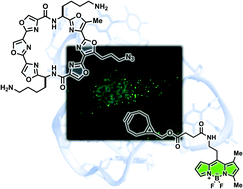
Macrocyclic hexaoxazoles (6OTDs) are G-quadruplex (G4) ligands, and some derivatives, such as L2H2-6OTD (1a) bearing two aminobutyl side chains, show cytotoxicity towards cancer cells. To identify the cellular target of 1a, we employed a post-target-binding strategy utilizing click reaction (Huisgen cyclization) between the azide-conjugated ligand L2H2-6OTD-Az (1b) and the cell-permeable dye CO-1 bearing a strained alkyne moiety and the BODIPY fluorophore under Cu-free conditions. We confirmed that introduction of the small azide group did not alter the physical or biological properties, including anti-cancer activity, of 1a, and we also demonstrated bias-free localization of CO-1. The post-binding visualization strategy suggested that L2H2-6OTD (1a) colocalized with RNA G4 in living cells.


 Please wait while we load your content...
Please wait while we load your content...