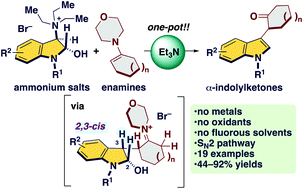
A metal-, oxidant-, and fluorous solvent-free synthesis of α-indolylketones enabled by an umpolung strategy†
Abstract
Enamines undergo α-indolization with ammonium salts in the presence of Et3N to form α-indolylketones. This is the first example of transition metal-, oxidant-, and fluorous solvent-free α-indolization of ketones. Key to the success of this convenient protocol was the use of in situ generated electrophilic indole species, as well as the use of enamines as a ketone enolate equivalent.


 Please wait while we load your content...
Please wait while we load your content...