Weak coordinated nitrogen functionality enabled regioselective C–H alkynylation via Pd(ii)/mono-N-protected amino acid catalysis†
Abstract
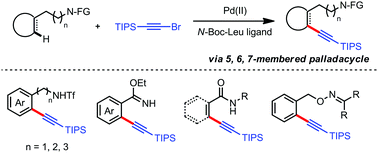
The exploration of weak coordinated amine derivative enabled regioselective C–H functionalization remains challenging due to the elusive achievement of reactivity and selectivity simultaneously. Herein, regioselective C–H alkynylation of various readily transformable nitrogen functionalities was developed with great efficiency, with the assistance of the mono-N-protected amino acid (MPAA) ligand via Pd(II) catalysis proceeding via 5, 6 and 7-membered palladacycle intermediates.


 Please wait while we load your content...
Please wait while we load your content...