Multiple applications of polymers containing electron-reservoir metal-sandwich complexes†
Abstract
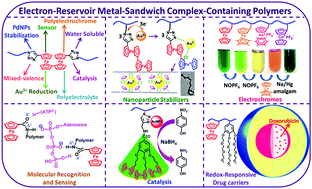
Ferrocene-containing polymers have been investigated for more than six decades, and more recently modern synthetic methods have allowed the fabrication of precise polymers that contain a variety of transition-metal complexes. Trends are now oriented towards applications, such as optics, energy conversion and storage, electrochemistry, magnetics, electric conductors and biomedicine. Metal-sandwich complexes such as those of ferrocene type and other related complexes that present redox-robust groups in polymers, i.e. that are isolable in both their oxidized and reduced forms, are of particular interest, because it is possible to address them using electronic or photonic redox stimuli for application. Our research groups have called such complexes Electron-Reservoirs and introduced them in the main chain or in the side chains of well-defined polymers. For instance, polymers with ferrocene in the main chain or in the side chain are oxidized to stable polycationic polyelectrolytes only if ferrocene is part of a biferrocene unit, because biferrocene oxidation leads to the biferrocenium cation that is stabilized by the mixed valency. Then a group of several redox-robust iron sandwich complexes were fabricated and incorporated in precise polymers including multi-block copolymers whose controlled synthesis and block incorporation was achieved for instance using ring-opening-metathesis polymerization. Applications of this family of Electron-Reservoir-containing polymers includes electrochemically induced derivatization of electrodes by decorating them with these polymers, molecular recognition and redox sensing, electrochromics with multiple colours, generation of gold and silver nanoparticles of various size by reduction of gold(III) and silver(I) precursors and their use for nanocatalysis towards depollution and biomedicine.


 Please wait while we load your content...
Please wait while we load your content...