Fourfold symmetric MCR's via the tetraisocyanide 1,3-diisocyano-2,2-bis(isocyanomethyl)propane†
Abstract
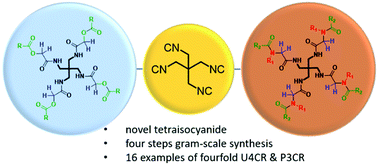
Oligoisocyanides are attractive synthetic targets, however, only a few are known. Here, we describe the smallest stable tetraisocyanide possible, the 1,3-diisocyano-2,2-bis(isocyano-methyl)propane (1) with S4 symmetry. Its four-step synthesis, structure, and reactivity in unprecedented symmetric fourfold Ugi 4CR and fourfold Passerini 3CR are described. Exhibiting high functional group tolerance and moderate to high yields, we foresee multiple applications of 1,3-diisocyano-2,2-bis(isocyanomethyl)propane, for example in MOFs, COFs, dendrimers, or artificial organs.


 Please wait while we load your content...
Please wait while we load your content...