Highly enantioselective addition of aliphatic aldehydes to 2-hydroxychalcone enabled by cooperative organocatalysts†
Abstract
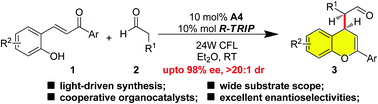
Herein, we developed an enantioselective addition of aliphatic aldehydes to 2-hydroxychalcone promoted by cooperative organocatalysts, giving access to hybrid flavonoids in excellent enantioselectivities. This reaction took advantage of cycloisomerization of 2-hydroxychalcone to form a transient flavylium under the irradiation of 24 W CFL, which was trapped by the in situ generated chiral enamine intermediate. The synergistic action of chiral phosphoric acid secured the excellent outcome of this reaction by ion-pairing with the transient flavylium.


 Please wait while we load your content...
Please wait while we load your content...