Enantioselective zinc-mediated conjugate alkynylation of saccharin-derived 1-aza-butadienes†
Abstract
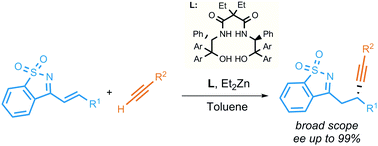
The enantioselective 1,4-alkynylation of conjugated imines derived from saccharin with aryl- and alkyl-substituted terminal alkynes has been achieved. The reaction mediated by diethylzinc in the presence of a catalytic amount of a bis(hydroxy)malonamide chiral ligand provides the corresponding imines bearing a propargylic stereocenter with moderate yields and fair to excellent enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...