Lewis acid–base synergistic catalysis of cationic halogen-bonding-donors with nucleophilic counter anions†
Abstract
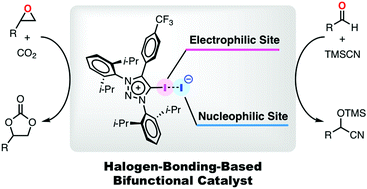
Cationic halogen-bonding-donors with little or non-coordinating counter anions have attracted great attention as new Lewis acid type organocatalysts. However, these anions cannot function as nucleophilic activation sites due to their low Lewis basicity. In this study, 1,3,4-triaryl-5-iodotriazolium iodides have been developed as bifunctional catalysts for simultaneous activation of nucleophiles and electrophiles. Computational and experimental studies indicated that the nucleophilicity of the counter anions plays an important role in achieving high catalytic efficiency for the cyanosilylation of aldehydes. In addition, the first report on carbon dioxide fixation by XB-donors is described.


 Please wait while we load your content...
Please wait while we load your content...