Synthesis of a porphyrin with histidine-like chelate: an efficient path towards molecular PDT/SPECT theranostics†
Abstract
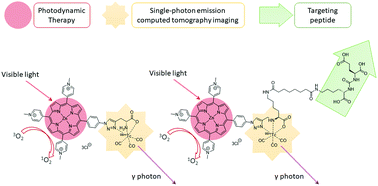
The goal of “personalised” medicine has seen a growing interest in the development of theranostic agents. Bifunctional, and targeted-trifunctional, theranostic water-soluble porphyrins with a histidine-like chelating group have been synthesised via copper-catalysed azide–alkyne cycloaddition (CuAAC) “click” chemistry in high yield and purity. They are capable of photodynamic treatment and [99mTc(CO)3]+ complexation for single-photon emission computed tomography (SPECT) imaging, with a radiochemical yield of >95%. The toxicity and phototoxicity were evaluated on HT-29 cells, DU145, and DU145-PSMA cell lines, with the targeted theranostic showing more potent phototoxicity towards DU145-PSMA expressing cells.


 Please wait while we load your content...
Please wait while we load your content...