Synthesis of di(hetero)aryl sulfides by defluorinative sulfenylation of polyfluoroalkyl ketones with sodium sulfinates or arylsulfonyl chlorides†
Abstract
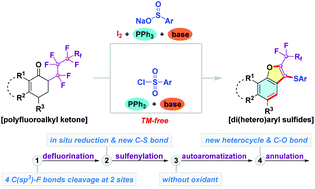
A facile incorporation of a privileged sulfide, a naphthofuran framework, and a perfluoroalkyl moiety in one molecule was successfully accomplished through tandem defluorinative sulfenylation of α-perfluoroalkyl ketones with a sulfur source. The reaction presumably proceeds via a sequence involving defluorination, reductive sulfenylation, autoaromatization, and annulation, accompanied by the simultaneous cleavage of four C(sp3)–F bonds and the formation of new C–S and C–O bonds.


 Please wait while we load your content...
Please wait while we load your content...