Trisubstituted geminal diazaalkene derived transient 1,2-carbodications†
Abstract
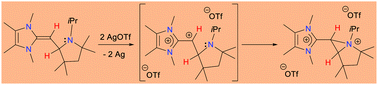
The coulombic repulsion between two adjacent cation centres of 1,2-carbodications is known to decrease with π- and/or n-donor substituents by a positive charge delocalization. Here we report the delocalization of the positive charge of transient 1,2-carbodications having one H-substituent by an intramolecular base-coordination. N-heterocyclic olefin (NHO) derived 2-pyrrolidinyl appended trisubstituted geminal diazaalkenes were used for the generation of transient 1,2-carbodications through a 2-e chemical oxidation process. We have also studied the 1-e oxidation reaction of trisubstituted geminal diazaalkenes (electrochemically and chemically) and also studied them using in situ EPR spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...