Lewis acid catalyzed reactivity switch: pseudo three-component annulation of nitrosoarenes and (epoxy)styrenes†
Abstract
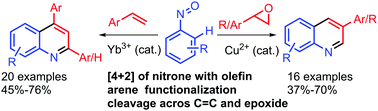
A Lewis acid catalyzed annulation reaction via arene functionalization of nitrosoarenes and C–C cleavage of (epoxy)styrene to provide arylquinolines is reported. The Lewis acid catalyst altered the annulation pattern providing arylquinolines instead of oxazolidines. The reaction with styrene resulted in a mixture of 2,4-diarylquinoline and 4-arylquinoline, while only 3-arylquinoline was formed from the reaction of epoxystyrene.


 Please wait while we load your content...
Please wait while we load your content...