Bimolecular vinylation of arenes by vinyl cations†
Abstract
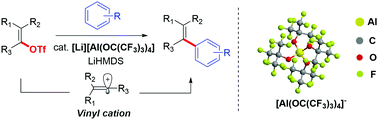
Styrene derivatives can be easily synthesized from vinyl triflates and arenes under mild reaction conditions, using [Li][Al(OC(CF3)3)4] as a catalyst and LiHMDS as a base. This transformation is likely to involve a vinyl cation intermediate as an electrophile, which is corroborated by DFT calculations, deuterium-labeling and other control experiments. The use of an inert weakly coordinating anion is a decisive factor in this bimolecular vinylation process.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...