Sustainable synthesis of 1,2,3,4-cyclohexanetetracarboxylate from sugar-derived carboxylic acids†
Abstract
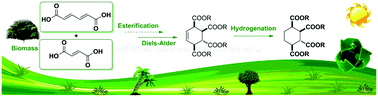
Herein, we report a sustainable route for the synthesis of 1,2,3,4-cyclohexanetetracarboxylate from sugar-derived muconic acid and fumaric acid. The key Diels–Alder reaction constructed a cyclohexene framework substituted by four ester groups. The isolated yield of tetramethyl 5-cyclohexene-1,2,3,4-tetracarboxylate was up to 95.5% without any catalyst used. And the hydrogenation reaction of the cycloadduct was catalyzed by commercial RANEY® Ni at room temperature and nearly 100% yield of the cyclohexyl target products was obtained.


 Please wait while we load your content...
Please wait while we load your content...