Supramolecular organogel formation through three-dimensional α-cyclodextrin nanostructures: solvent chirality-selective organogel formation†
Abstract
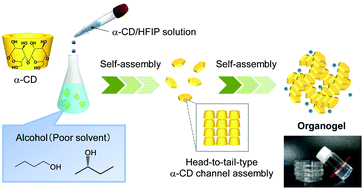
Novel supramolecular organogels were efficiently formed by mixing a 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) solution of α-cyclodextrin (α-CD) with 1- or 2-butanol via the formation of three-dimensional hexagonal nanostructures composed of head-to-tail α-CD channel assemblies. Mixing (R)- and (S)-2-butanol with an α-CD/HFIP solution realized (S)-2-butanol-selective organogel formation.


 Please wait while we load your content...
Please wait while we load your content...