Copper catalysed oxidative α-sulfonylation of branched aldehydes using the acid enhanced reactivity of manganese(iv) oxide†
Abstract
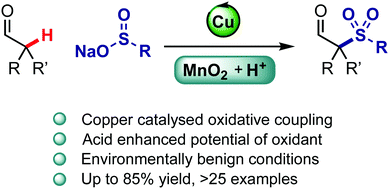
The oxidative coupling of secondary aldehydes and sulfinate salts is achieved using copper catalysis to form α-sulfonyl aldehydes. The use of an acidic co-solvent is important to adjust the oxidation potential of MnO2 as an oxidant. A broad range of sulfonylated aldehydes is prepared, and their further functionalisation is demonstrated. A dual ionic/radical pathway mechanism is proposed.


 Please wait while we load your content...
Please wait while we load your content...