Control of the toxic conformation of amyloid β42 by intramolecular disulfide bond formation†
Abstract
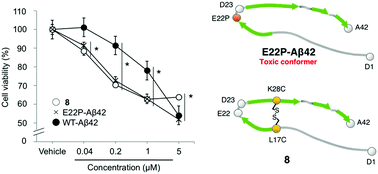
We report a method to fix the conformation of Aβ42 to the toxic or non-toxic form by intramolecular disulfide bonds. We found that an Aβ42 analog crosslinked within the molecule at the 17th and 28th amino acid residues exhibited high aggregative ability and potent neurotoxicity comparable to those of E22P-Aβ42. This analog would be useful in the research of Aβ42 oligomers and to develop reliable antibodies for early diagnosis of Alzheimer's disease.


 Please wait while we load your content...
Please wait while we load your content...