Metal free amination of congested and functionalized alkyl bromides at room temperature†
Abstract
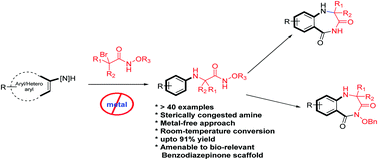
Herein, we report a highly facile and unprecedented approach to synthesize congested N-(hetero)aryl amines en route to α-amino acid amides using α-bromoamides as alkylating agents under mild reaction conditions (room temperature). The involvement of aza-oxyallyl cations as alkylating agents is the hallmark of this reaction. The method was readily adapted for the rapid synthesis of coveted 1,4-benzodiazepine-3,5-diones.


 Please wait while we load your content...
Please wait while we load your content...