Directed evolution of Anabaena variabilis phenylalanine ammonia-lyase (PAL) identifies mutants with enhanced activities†
Abstract
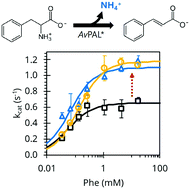
There is broad interest in engineering phenylalanine ammonia-lyase (PAL) for its biocatalytic applications in industry and medicine. While site-specific mutagenesis has been employed to improve PAL stability or substrate specificity, combinatorial techniques are poorly explored. Here, we report development of a directed evolution technique to engineer PAL enzymes. Central to this approach is a high-throughput enrichment that couples E. coli growth to PAL activity. Starting with the PAL used in the formulation of pegvaliase for PKU therapy, we report previously unidentified mutations that increase turnover frequency almost twofold after only a single round of engineering.


 Please wait while we load your content...
Please wait while we load your content...