Diamine-mediated N2-selective β-selenoalkylation of triazoles with alkenes†
Abstract
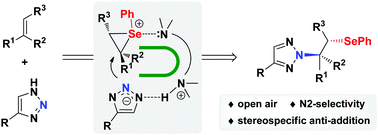
A N2-selective β-selenoalkylation of 1,2,3-triazoles with alkenes mediated by diamines has been developed. The reaction proceeds presumably via the interaction of diamines with both the triazole moiety and selenium/alkene complex to construct a U-shaped reaction intermediate. This activation mode will block the N1 position on triazoles and thus favor the N2-selective selenoamination. This stereospecific anti-addition method enables an efficient N2-selective β-selenoalkylation of 1,2,3-triazoles under mild and open-air conditions and might find applications in the synthesis of biologically active molecules.


 Please wait while we load your content...
Please wait while we load your content...